INTRODUCTION
Systemic opioids are considered an effective treatment option for patients with post-surgical spine syndrome (PSSS) in whom severe pain cannot be controlled despite general treatment, such as drug therapy and nerve block, for pain relief. However, it can cause several side effects. Intrathecal morphine (ITM) treatment can reduce drug interventions, minimize side effects, and increase therapeutic effects. Morphine is a hydrophilic opioid analgesic used to treat acute and chronic pain; thus, when injected intrathecally, morphine spreads gradually through the cerebrospinal fluid (CSF) circulation pathway, including the periaqueductal-periventricular gray matter, ventromedial medulla, and mu-receptors in the spinal cord, rapidly binding to several opiate receptors and ion channel. It can also suppress pain and produce an adequate analgesic effect, even at low doses. In animal experiments, morphine administered directly into the subarachnoid space of the rat spine induces strong analgesic inhibition [1]. On this basis, clinical studies on the use of ITM to treat obstetric and refractory pain are currently being performed [2,3]. However, aside from the positive effects of ITM, side effects have also been reported [4-9], including respiratory depression, nausea, vomiting, hypotension, bradycardia, pruritus, and urinary retention [10]. Although morphine-related side effects are the most common with ITM treatment, device- or procedure-related complications may also rarely occur [7-9]. In this study, patients with PSSS who underwent an ITM trial or pump implantation were classified into three categories—drug side effects, instrument, and procedure-related complications—and were analyzed.
MATERIALS AND METHODS
Overall, 26 patients with PSSS who underwent the ITM trial or pump implantation were enrolled. The patients included 25 men and 1 woman, the mean age and standard deviation (STD) was 61.5±13.4 years (range, 37–82 years), and the follow-up period ranged from 3 to 3,690 days. The average follow-up duration for patients who underwent only the ITM trial was 5.4 days (range: 3–14 days), with an average of 55.9 months (range: 15–123 months) for those who underwent ITM pump (ITMP) implantation. The ITM trial was conducted on patients who experienced pain with a visual analog scale (VAS) score of 7 or higher for more than 6 months, despite various treatments for pain relief, such as drug therapy and nerve block. The ITMP implantation was performed in patients with a decrease in the VAS score of 50% or more after the ITM trial. We used Highmol (Bio & Chemical R&D, Korea), a morphine free of preservatives, and determined the dose considering the degree of pain relief and occurrence of side effects. The ITM trial was performed with patients in the lateral decubitus position. After skin preparation and draping, a 25-gauge 10-cm spinal needle was inserted into the L2–3, L3–4, or L4–5 interspinous space to check the CSF flow. Subsequently, a bolus of 0.1–1 mg Highmol and 0.9% saline mixed solution was injected intrathecally over 5–10 seconds, and the spinal needle was removed. The trial was the only procedure performed in 19 of the 26 patients. The remaining 8 patients had a VAS score that did not decrease by more than 50%, of whom all 8 had urinary retention, and 3 had pruritus. After the ITM trial, no further ITM administration or pump implantation was performed if side effects occurred or if the VAS score was not reduced by more than 50%. The 7/26 patients in whom the VAS score decreased by less than 50%, and no ITM-related side effects related to the trial were experienced underwent ITMP implantation for continuous morphine injection to control the pain. ITMP implantation was performed with the patients in the lateral position under general anesthesia. After we performed dural puncture at the L2–3, L3–4, or L4–5 interspinous area and checked for CSF flow, the catheter tip was inserted up to L1–2 or T7–10, and the catheter was anchored to the fascia of the paraspinal muscle and fixed. A morphine pump pocket was created in the abdomen, an implantable pulse generator (IPG) was inserted, and the IPG and distal catheter were connected via subcutaneous tunneling.
RESULTS
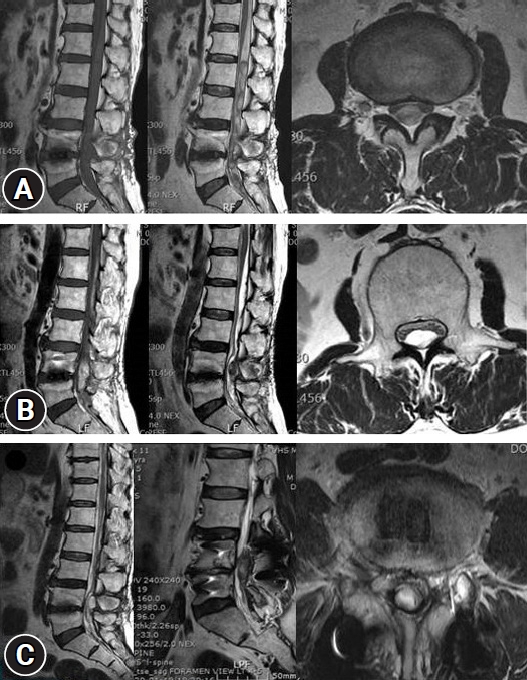
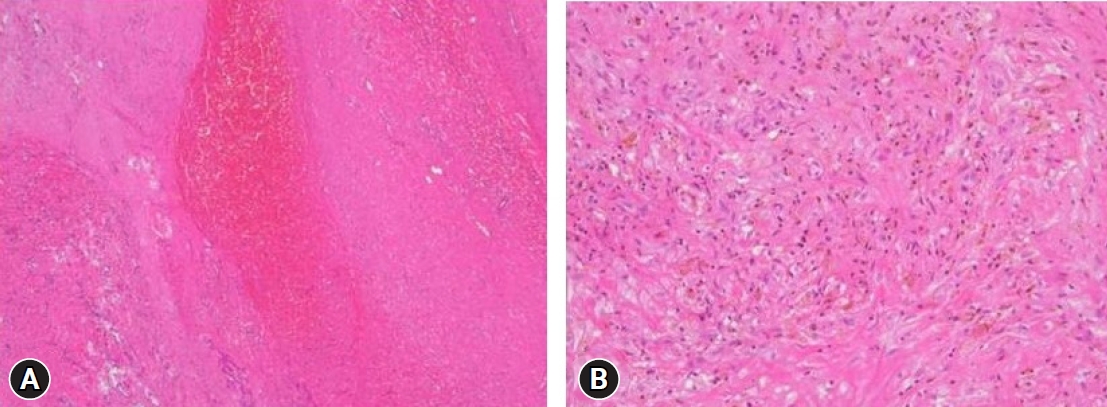
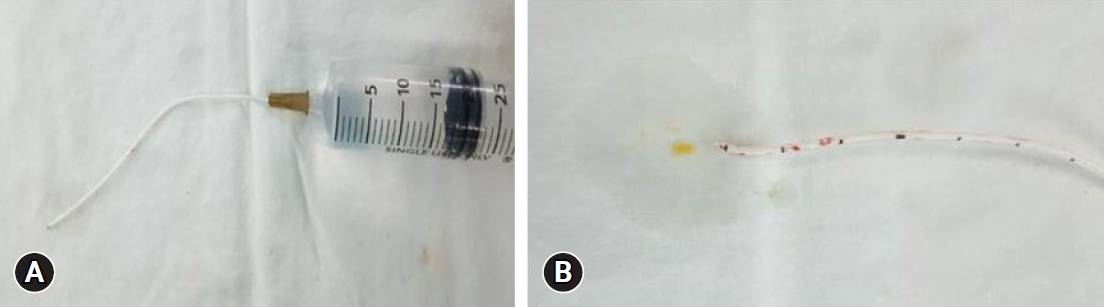
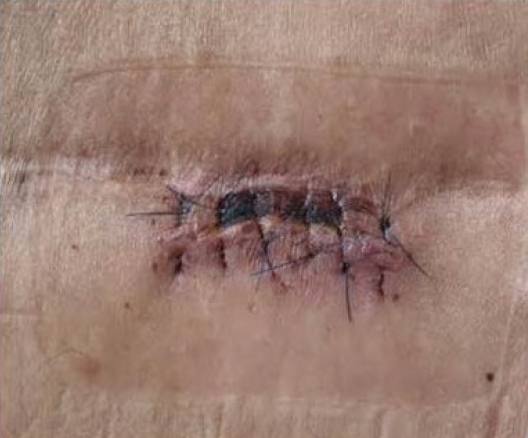
Regarding the history of spinal surgery, there were 24 cases in the lumbar and sacral region, 1 in the thoracic vertebrae, and 1 in the cervical vertebrae. There were 4 cases of lumbar and sacral decompression, 20 cases of decompression and fusion, 1 case of thoracic decompression and fixation, and 1 case of cervical decompression and fixation. Of the 26 patients, 13 (50%) experienced no side effects or complications related to the ITM trial or the ITMP implantation. The most common complications included temporary drug side effects in 11 patients (42.3%): 8 (30.8%) had difficulty in urination and 3 (11.5%) had pruritus (Table 1). A single bolus of 0.25–1.5 mg ITM was injected in patients with urinary retention, and 0.1–1.0 mg was injected in patients without urinary retention. The difference in the amount of the single bolus administered in the two groups was small and not proportional to the urination difficulty. After discontinuing ITM and injecting naloxone instead, urinary retention improved within 2–5 days. In patients with pruritis, a single bolus of 0.3–0.5 mg ITM was injected, and pruritis improved within 1–2 days after ITM was stopped and an antihistamine was administered. After several ITM trials, there was one case of a large lumbosacral subdural hematoma (SDH) with caudal equina fibrosis (Fig. 1, 2), and another case in which the middle of the catheter was blocked by a precipitated morphine crystal and the IPG was discharged at the same time, and so the ITM pump did not work (Fig. 3). In a patient with an ITMP implantation, a part of the back skin in the incision was necrotized (Fig. 4, Table 2). Seven patients who underwent ITMP implantation with a VAS score of 7 or higher finally experienced a reduction in their VAS score by 2–4 values, but they still had moderate pain with a VAS score of 4–6.
DISCUSSION
Adverse events, causes, and consequences have all been reported to occur as a result of ITM injection [4-9]. Pendi et al. [4] reported that the use of ITM in spine surgery significantly lowered postoperative morphine consumption and VAS score, and was shown to increase pruritus for 24 hours after surgery in a meta-analysis of eight randomized controlled trials including 393 patients in 2017. In our study, we analyzed the morphine-related side effects or complications that occurred in patients who underwent the ITM trial or pump implantation. Of these 26 patients, 13 (50%) had no morphine-related side effects or complications. Regarding ITM, 11 (42.3%) of the 26 patients had drug-related complications, of which urinary retention was the most common, occurring in 8 patients (30.7%), followed by pruritus in 3 patients (11.5%). Urinary retention and pruritus were temporary adverse reactions that improved with conservative treatment. Overall, 23 patients (88.5%) had no or only transient adverse reactions, and most of them did not have serious side effects from the ITM trial. According to Ruan [5], most drug-related side effects with long-term ITM therapy are opioid receptor-mediated, and nausea, vomiting, pruritus, urinary retention, constipation, and sexual dysfunction are the most common. Although relatively uncommon, respiratory depression and hyperalgesia may also reportedly occur [5]. Urinary retention develops in 42%–80% of patients and is more common in older patients with an enlarged prostate [11,12]. The underlying mechanism of action involves stimulation of the detrusor muscle by the sacral parasympathetic fiber to induce bladder contraction. This inhibits sacral parasympathetic outflow via an interaction with opioid receptors of the sacral cord, particularly the mu and delta receptors, and induces urinary retention through detrusor relaxation [5,13]. Pruritus is one of the common side effects that can appear on the face, neck, and upper chest [14,15]. It develops several hours after drug administration and is usually mild [12]. Owing to morphine’s high hydrophilicity, it exhibits cephalad migration in the CSF. Its interaction with the trigeminal nucleus of the medulla reportedly causes an itch reflex by connecting with the substantia gelatinosa of the dorsal horn, and can induce changes in central nervous system-related pain perception [16,17]. According to one study, the analgesic effect is maintained and pruritus can be improved when an intravenous drip of 5 μg/kg/hr naloxone is administered, and rapid tolerance occurs after 1–2 weeks [5]. Although there were no cases in this study, respiratory depression is one of the most dangerous complications of ITM treatment [18,19], and CSF reportedly interacts with the ventral medulla through cephalad migration and suppresses the respiratory center [20].
According to Kim et al. [7], catheter-related complications include catheter migration, fracture, occlusion, drug-related complications, and surgery-related complications, such as hematomas. Intradural inflammatory granulomas occurring at the tip of the IT catheter are an example of catheter-related complications. A unique finding of this study was the discovery of precipitated nonpolarizable crystals during pathological examination of the removed granuloma tissue, which were identified as precipitated morphine by liquid extraction surface analysis [7]. In this study, catheter-tip granuloma did not occur, but there was one case (4%) in which the ITMP did not work properly because the IPG was discharged and the mid-catheter was simultaneously blocked with morphine crystals. In this case, the patient was receiving 2.0–2.5 mg/d of morphine six years after ITMP implantation; however, when the ITMP failed, he experienced abrupt aggravated pain. No abnormalities were found on X-ray or computed tomography (CT) scans. When we confirmed that the motor did not work in the device, we attempted contrast medium injection and CSF aspiration in the side port of the pump, but failed and assumed catheter obstruction. When the IT catheter was removed and visually checked, no side-hole obstruction could be observed (Fig. 3A). However, when saline was injected into the catheter using a syringe, a yellow crystalline material was ejected (Fig. 3B), and later identified as morphine crystals. Bolash et al. [21] previously reported that the pump could be used for an average of 5.9 years. However, if removal is required earlier than the average usable lifespan for reasons such as infection, reduced treatment effectiveness, surgical complications, and granuloma formation, the average lifespan is shortened to 5.4 years [21]. If the ITMP does not work even though the ITM is replenished at the optimal time, the IPG and catheter should be checked for abnormalities, as the IPG life may have expired or the catheter may be clogged.
Surgical complications should also be considered in such cases. Patients with PSSS are more likely to undergo reoperation than patients with other spinal surgeries; therefore, their skin condition is poor and there is a high risk of postoperative infection or skin necrosis. In this study, skin lesions occurred in two cases (7.7%) related to surgery, and abdominal skin infection and back skin necrosis occurred in one case each. Abdominal skin infection was observed in the patient who underwent repeated IPG replacement, and was treated with antibiotics without IPG removal. In the patient with multiple lumbar decompression and fixation, part of the back skin in the incision became necrotized. However, the skin regenerated and could be treated with skin dressing alone.
Knight et al. [8] previously reported that bleeding may occur owing to inappropriate hemostasis during the procedure, and if it progresses seriously, neurological symptoms and deterioration of back pain may occur. In this study, to determine whether ITM is effective in controlling pain, intradural morphine trials were performed in the same area several times. As a result, we found that subdual bleeding and inflammation gradually progressed, resulting in a large subdural hematoma (SDH) and caudal equina fibrosis and granulation. Large lumbosacral SDH developed, and the cauda equina was severely compressed, resulting in neurological adverse reactions such as muscle weakness and sensory abnormalities in the lower extremities. Surgery was performed to remove the SDH of the lumbar spine. Ichinose et al. [9] previously reported a case of rare intracranial and lumbar chronic SDH that was treated with lumbar puncture and burr hole surgery. In this study, there were two cases of severe complications, including catheter blockage and a large SDH. Thus, patients should be diagnosed with neurological tests, device evaluations, and imaging tests, such as CT and magnetic resonance imaging, and surgical treatment should be considered in accordance with the test results.
CONCLUSION
Urination difficulties are common in ITM trials, but usually resolve within a few days. Other complications, such as a large lumbosacral SDH or catheter blockage, may occur. Complications can be reduced by understanding the pharmacological mechanisms, equipment, and techniques related to ITM.